Polyvinyl chloride
From Wikipedia, the free encyclopedia
"PVC" redirects here. For other uses, see PVC (disambiguation).
| Polyvinyl chloride | |
|---|---|
 | |
 | |
 | |
| Identifiers | |
| Abbreviations | PVC |
| CAS number | 9002-86-2 |
| KEGG | C19508 |
| MeSH | Polyvinyl+Chloride |
| ChEBI | CHEBI:53243 |
| Properties | |
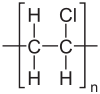
| Molecular formula | (C2H3Cl)n[2] |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
| Infobox references | |
| Elongation at break | 20–40% |
|---|---|
| Notch test | 2–5 kJ/m2 |
| Glass temperature | 82 °C[3] |
| Melting point | 100–260 °C[3] |
| Effective heat of combustion | 17.95 MJ/kg |
| Specific heat (c) | 0.9 kJ/(kg·K) |
| Water absorption (ASTM) | 0.04–0.4 |
| Dielectric Breakdown Voltage | 40 MV/m |
Polyvinyl chloride, commonly abbreviated PVC, is the third-most widely produced plastic, afterpolyethylene and polypropylene.[4] PVC is used inconstruction because it is more effective than traditional materials such as copper, iron or wood in pipe and profile applications. It can be made softer and more flexible by the addition of plasticizers, the most widely used being phthalates. In this form, it is also used in clothing and upholstery, electrical cable insulation, inflatable products and many applications in which it replaces rubber.[5]
Pure polyvinyl chloride is a white, brittle solid. It is insoluble in alcohol, but slightly soluble intetrahydrofuran.
Contents
[hide]Discovery and production
PVC was accidentally discovered at least twice in the 19th century, first in 1835 by French chemistHenri Victor Regnault and then in 1872 by German chemist Eugen Baumann. On both occasions the polymer appeared as a white solid inside flasks of vinyl chloride that had been left exposed to sunlight. In the early 20th century the Russian chemist Ivan Ostromislensky and Fritz Klatte of the German chemical company Griesheim-Elektron both attempted to use PVC in commercial products, but difficulties in processing the rigid, sometimes brittle polymer blocked their efforts. Waldo Semonand the B.F. Goodrich Company developed a method in 1926 to plasticize PVC by blending it with various additives. The result was a more flexible and more easily processed material that soon achieved widespread commercial use.
About 80% of production involves suspension polymerization. Emulsion polymerization accounts for about 12% and bulk polymerization accounts for 8%. Suspension polymerizations affords particles with average diameters of 100 – 180 μm, whereas emulsion polymerization gives much smaller particles of average size around 0.2 μm. VCM and water are introduced into the reactor and a polymerization initiator, along with other additives. The reaction vessel is pressure tight to contain the VCM. The contents of the reaction vessel are continually mixed to maintain the suspension and ensure a uniform particle size of the PVC resin. The reaction is exothermic, and thus requires cooling. As the volume is reduced during the reaction (PVC is denser than VCM), water is continually added to the mixture to maintain the suspension.[4]
The polymerization of VCM is started by compounds called initiators that are mixed into the droplets. These compounds break down to start the radical chain reaction. Typical initiators include dioctanoylperoxide and dicetyl peroxydicarbonate, both of which have fragile O-O bonds. Some initiators start the reaction rapidly but decay quickly and other initiators have the opposite effect. A combination of two different initiators is often used to give a uniform rate of polymerization. After the polymer has grown by about 10x, the short polymer precipitates inside the droplet of VCM, and polymerization continues with the precipitated, solvent-swollen particles. The weight average molecular weights of commercial polymers range from 100,000 to 200,000 and the number average molecular weights range from 45,000 to 64,000.
Once the reaction has run its course, the resulting PVC slurry is degassed and stripped to remove excess VCM, which is recycled. The polymer is then passed though a centrifuge to remove water. The slurry is further dried in a hot air bed, and the resulting powder sieved before storage or pelletization. Normally, the resulting PVC has a VCM content of less than 1 part per million. Other production processes, such as micro-suspension polymerization and emulsion polymerization, produce PVC with smaller particle sizes (10 μm vs. 120–150 μm for suspension PVC) with slightly different properties and with somewhat different sets of applications.
Microstructure
The polymers are linear and are strong. The monomers are mainly arranged head-to-tail, meaning that there are chlorides on alternating carbon centres. PVC has mainly an atactic stereochemistry, which means that the relative stereochemistry of the chloride centres are random. Some degree ofsyndiotacticity of the chain gives a few percent crystallinity that is influential on the properties of the material. About 57% of the mass of PVC is chlorine. The presence of chloride groups gives the polymer very different properties from the structurally related material polyethylene.[7]
Additives to finished polymer
The product of the polymerization process is unmodified PVC. Before PVC can be made into finished products, it always requires conversion into a compound by the incorporation of additives such as heat stabilizers, UV stabilizers, lubricants, plasticizers, processing aids, impact modifiers, thermal modifiers, fillers, flame retardants, biocides, blowing agents and smoke suppressors, and, optionally pigments.[8] The choice of additives used for the PVC finished product is controlled by the cost performance requirements of the end use specification e.g. underground pipe, window frames, intravenous tubing and flooring all have very different ingredients to suit their performance requirements.
Phthalate plasticizers
Most vinyl products contain plasticizers which dramatically improve their performance characteristic. The most common plasticizers are derivatives of phthalic acid. The materials are selected on their compatibility with the polymer, low volatility levels, and cost. These materials are usually oily colourless substances that mix well with the PVC particles. 90% of the plasticizer market, estimated to be millions of tons per year worldwide, is dedicated to PVC.[8]
High and low molecular weight phthalates
Phthalates can be divided into two groups: high and low molecular weight, with high molecular weight phthalates now representing over 80 percent of European market for plasticisers. Low molecular weight phthalates include those with 3-6 carbon atoms in their chemical backbone; the most common types being Di(2-ethylhexyl) phthalate (DEHP), Di-butyl phthalate (DBP), Di- isobutyl phthalate (DIBP) and Butyl benzyl phthalate (BBP). Because of possible health effects of low phthalates in the environment, including Di(2-ethylhexyl) phthalate, there is movement to replace them with safer alternatives in Canada, the European Union, and the United States. They represent about 15% of the European market. High molecular weight phthalates include those with 7-13 Carbon atoms in their chemical backbone, which gives them increased permanency and durability. The most common types of high phthalates include di-isononyl phthalate (DINP) and di-isodecyl phthalate (DIDP). The European market has been shifting in the last decade from low to high phthalates, which today represent over 80% of all the phthalates currently being produced in Europe.
Heat stabilizers
One of the most crucial additives are heat stabilizers. These agents minimize loss of HCl, a degradation process that starts above 70 °C. Once dehydrochlorination starts, it is autocatalytic. Many diverse agents have been used including, traditionally, derivatives of heavy metals (lead, cadmium). Increasingly, metallic soaps (metal "salts" of fatty acids) are favored, species such ascalcium stearate.[4] Addition levels vary typically from 2% to 4%. The choice of the best heat stabilizer depends on its cost effectiveness in the end use application, performance specification requirements, processing technology and regulatory approvals.
Rigid PVC Applications
In Europe there has been a commitment to eliminate the use of cadmium (previously used as a part component of heat stabilizers in window profiles) and phase out lead based heat stabilizers (as used in pipe and profile areas) by 2015. According to the final report of Vinyl 2010[9] cadmium was eliminated across Europe by 2007. The progressive substitution of lead-based stabilizers is also confirmed in the same document showing a reduction of 75% since 2000 and ongoing. This is confirmed by the corresponding growth in calcium-based stabilizers, used as an alternative to lead-based stabilizers, more and more, also outside Europe.
Tin based stabilizers are mainly used in Europe for rigid, transparent applications due to the high temperature processing conditions used. The situation in North America is different where tin systems are used for almost all-rigid PVC applications. Tin stabilizers can be divided into two main groups, the first group containing those with tin-oxygen bonds and the second group with tin-sulphur bonds. According to the European Stabiliser producers[10] most organotin stabilisers have already been successfully REACH registered. More chemical and use information is also available on this site.
Flexible PVC Applications
Flexible PVC coated wire and cable for electrical use has traditionally been stabilised with lead but these are being replaced, as in the rigid area, with calcium based systems.
Liquid mixed metal stabilisers are used in several PVC flexible applications such as calendered films, extruded profiles, injection moulded soles and footwear, extruded hoses and plastisols where PVC paste is spread on to a backing (flooring, wall covering, artificial leather). Liquid mixed metal stabiliser systems are primarily based on Barium, Zinc and Calcium carboxylates. In general liquid mixed metals like BaZn, CaZn require the addition of co-stabilisers, antioxidants and organo-phosphites to provide optimum performance.
BaZn stabilisers have successfully replaced Cadmium-based stabilisers in Europe in many PVC semi-rigid and flexible applications according to the European producers.[11]
Physical properties
PVC is a thermoplastic polymer. Its properties for PVC are usually categorized based on rigid and flexible PVCs.
| Property | Rigid PVC | Flexible PVC |
|---|---|---|
| Density [g/cm3][12] | 1.3–1.45 | 1.1–1.35 |
| Thermal conductivity [W/(m·K)][13] | 0.14–0.28 | 0.14–0.17 |
| Yield strength [psi][12] | 4500 - 8700 | 1450 - 3600 |
| Young's modulus [psi] | 490,000[14] | |
| Flexural strength (yield) [psi] | 10,500[14] | |
| Compression strength [psi] | 9500[14] | |
| Coefficient of thermal expansion (linear) [mm/(mm °C)] | 5×10−5[14] | |
| Vicat B [°C][13] | 65–100 | Not recommended |
| Resistivity [Ω m][15][16] | 1016 | 1012–1015 |
| Surface resistivity [Ω][15][16] | 1013–1014 | 1011–1012 |
Mechanical properties
PVC has high hardness and mechanical properties. The mechanical properties enhance with the molecular weight increasing, but decrease with the temperature increasing. The mechanical properties of rigid PVC (uPVC) is very good, the elastic modulus can reach to 1500-3,000 MPa. The soft PVC (Flexible PVC) elastic is 1.5-15 MPa. However, elongation at break is up to 200% -450%. PVC friction is ordinary, the static friction factor is 0.4-0.5, the dynamic friction factor is 0.23.[17]
Thermal properties
The heat stability of PVC is very poor, when the temperature reaches 140 °C PVC starts to decompose. Its melting temperature is 160 °C. The linear expansion coefficient of the PVC is small and has flame retardancy, the oxidation index is up to 45 or more. Therefore, the addition of a heat stabilizer during the process is necessary in order to ensure the product's properties.
Electrical properties
PVC is a polymer with good insulation properties but because of its higher polar nature the electrical insulating property is inferior to non polar polymers such as polyethylene and polypropylene.
As the dielectric constant, dielectric loss tangent value and volume resistivity are high, the corona resistance is not very good, it is generally suitable for medium or low voltage and low frequency insulation materials.
Applications
PVC's relatively low cost, biological and chemical resistance and workability have resulted in it being used for a wide variety of applications. It is used for sewerage pipes and other pipe applications where cost or vulnerability to corrosion limit the use of metal. With the addition of impact modifiers and stabilizers, it has become a popular material for window and door frames. By adding plasticizers, it can become flexible enough to be used in cabling applications as a wire insulator. It has been used in many other applications. PVC demand is likely to increase at an average annual rate of 3.9% over the next years.[18]
Pipes
Roughly half of the world's polyvinyl chloride resin manufactured annually is used for producing pipes for municipal and industrial applications.[19] In the water distribution market it accounts for 66% of the market in the US, and in sanitary sewer pipe applications, it accounts for 75%.[20] Its light weight, low cost, and low maintenance make it attractive. However, it must be carefully installed and bedded to ensure longitudinal cracking and overbelling does not occur. Additionally, PVC pipes can be fused together using various solvent cements, or heat-fused (butt-fusion process, similar to joining HDPEpipe), creating permanent joints that are virtually impervious to leakage.
In February, 2007 the California Building Standards Code was updated to approve the use ofchlorinated polyvinyl chloride (CPVC) pipe for use in residential water supply piping systems. CPVC has been a nationally accepted material in the US since 1982; California, however, has permitted only limited use since 2001. The Department of Housing and Community Development prepared and certified an environmental impact statement resulting in a recommendation that the Commission adopt and approve the use of CPVC. The Commission's vote was unanimous and CPVC has been placed in the 2007 California Plumbing Code.
In the United States and Canada, PVC pipes account for the largest majority of pipe materials used in buried municipal applications for drinking water distribution and wastewater mains.[21] Buried PVC pipes in both water and sanitary sewer applications that are 4 inches (100 mm) in diameter and larger are typically joined by means of a gasket-sealed joint. The most common type of gasket utilized in North America is a metal reinforced elastomer, commonly referred to as a Rieber sealing system.[22]
Electric cables
PVC is commonly used as the insulation on electrical cables; PVC used for this purpose needs to beplasticized.
In a fire, PVC-coated wires can form HCl fumes; the chlorine serves to scavenge free radicals and is the source of the material's fire retardance. While HCl fumes can also pose a health hazard in their own right, HCl dissolves in moisture and breaks down onto surfaces, particularly in areas where the air is cool enough to breathe, and is not available for inhalation.[23] Frequently in applications where smoke is a major hazard (notably in tunnels and communal areas) PVC-free cable insulation is preferred, such as low smoke zero halogen (LSZH) insulation. Any metal parts must not be mixed together during the raw material stage, as it may lead to EMI.
Unplasticized polyvinyl chloride (uPVC) for construction
uPVC, also known as rigid PVC, is extensively used in the building industry as a low-maintenance material, particularly in Ireland, the United Kingdom, and in the United States. In the USA it is known as vinyl, or vinyl siding.[24] The material comes in a range of colors and finishes, including a photo-effect wood finish, and is used as a substitute for painted wood, mostly for window frames and sills when installing double glazingin new buildings, or to replace older single-glazed windows. Other uses include fascia, and siding orweatherboarding. This material has almost entirely replaced the use of cast iron for plumbing anddrainage, being used for waste pipes, drainpipes,gutters and downspouts. uPVC does not contain phthalates, since those are only added to flexible PVC, nor does it contain BPA. uPVC is known as having strong resistance against chemicals, sunlight, and oxidation from water.[25]
Signs
Polyvinyl chloride is formed in flat sheets in a variety of thicknesses and colors. As flat sheets, PVC is often expanded to create voids in the interior of the material, providing additional thickness without additional weight and minimal extra cost (see Closed-cell PVC foamboard). Sheets are cut using saw and rotary cutting equipment. Plasticized PVC is also used to produce thin, colored, or clear, adhesive-backed films referred to simply as vinyl. These films are typically cut on a computer-controlled plotter or printed in a wide-format printer. These sheets and films are used to produce a wide variety of commercial signageproducts and markings on vehicles, e.g. car body stripes.
Clothing and furniture
PVC has become widely used in clothing, to either create a leather-like material or at times simply for the effect of PVC. PVC clothing is common in Goth, Punk,clothing fetish and alternative fashions. PVC is cheaper than rubber, leather, and latex which it is therefore used to simulate.
PVC fabric has a sheen to it and is waterproof so is used in coats, skiing equipment, shoes, jackets,aprons, and bags.
Healthcare
The two main application areas for single use medically approved PVC compounds are flexible containers and tubing: containers used for blood and blood components for urine or for ostomy products and tubing used for blood taking and blood giving sets, catheters, heart-lung bypass sets, haemodialysis set etc. In Europe the consumption of PVC for medical devices is approximately 85.000 tons every year. Almost one third of plastic based medical devices are made from PVC.[26] The reasons for using flexible PVC in these applications for over 50 years are numerous and based on cost effectiveness linked to transparency, light weight, softness, tear strength, kink resistance, suitability for sterilization and biocompatibility.
Plasticisers
DEHP (Di-2ethylhexylphthalate) has been medically approved for many years for use in such medical devices; the PVC-DEHP combination proving to be very suitable for making blood bags because DEHP stabilises red blood cells so minimising haemolysis (red blood cell rupture) However DEHP is coming under increasing pressure in Europe. The assessment of potential risks related to phthalates and in particular the use of DEHP in PVC medical devices has been subject to scientific and policy review by the European Union authorities. As of 21 March 2010, a specific labelling requirement has subsequently been introduced across the EU for all devices containing phthalates that are classified as CMR (carcinogenic, mutagenic or toxic to reproduction).[27] The label aims to enable healthcare professionals to use this equipment safely and, where needed, take appropriate precautionary measures for patients at risk of over-exposure. DEHP alternatives, which are gradually replacing it, are Adipates, Butyryltrihexylcitrate (BTHC), Cyclohexane-1,2-dicarboxylic acid, diisononylester (DINCH), Di(2-ethylhexyl)terephthalate, polymerics and trimellitic acid, 2-ethylhexylester (TOTM).
Flooring
Flexible PVC flooring is inexpensive and used in a variety of buildings covering the home, hospitals, offices, schools, etc. Complex and 3D designs are possible due to the prints that can be created which are then protected by a clear wear layer. A middle vinyl foam layer also gives a comfortable and safe feel. The smooth, tough surface of the upper wear layer prevents the build up of dirt which prevents microbes from breeding in areas that need to be kept sterile, such as hospitals and clinics.
See also: Vinyl composition tile
Other applications
PVC has been used for a host of consumer products of relatively smaller volume compared to the industrial and commercial applications described above. Another of its earliest mass-market consumer applications was to make vinyl records. More recent examples include wallcovering, greenhouses, home playgrounds, foam and other toys, custom truck toppers (tarpaulins), ceiling tiles and other kinds of interior cladding.
Chlorinated PVC
PVC can be usefully modified by chlorination, which increases its chlorine content to 67%. The new material has a higher heat resistance so is primarily used for hot water pipe and fittings, but it is more expensive and it is found only in niche applications, such as certain water heaters and certain specialized clothing. An extensive market for chlorinated PVC is in pipe for use in office building, apartment and condominium fire protection. CPVC, as it is called, is produced by chlorination of aqueous solution of suspension PVC particles followed by exposure to UV light which initiates the free-radical chlorination.[4]
Health and safety
| This section is empty. You can help by adding to it. (August 2013) |
Degradation
Degradation is a chemical change that drastically reduces the average molecular weight of the polymer. Since the mechanical integrity of plastics invariably depends on their high average molecular-weight, any significant extent of degradation inevitably weakens the material. Weathering degradation of plastics results in their surface embrittlement and microcracking, yielding microparticles that continue on in the environment, known as microplastics. Microplastics concentrate Persistent Organic Pollutants (POPs). The relevant distribution coefficients for common POPs are several orders of magnitude in favor of the plastic medium. Consequently, the microparticles laden with high levels of POPs can be ingested by organisms in the biosphere. Given the increased levels of plastic pollution of the environment, this is an important concept in understanding the food web.[28]
Plasticizers
Phthalates, which are incorporated into plastics as plasticizers comprise ∼70% of the U.S. plasticizer market; phthalates are by design not covalently bound to the polymer matrix, which makes them highly susceptible to leaching. Phthalates are contained in plastics at high percentages. For example, they can contribute up to 40% by weight to intravenous medical bags and up to 80% by weight in medical tubing.[29] Vinyl products are pervasive—including toys,[30] car interiors, shower curtains, and flooring—and initially release chemical gases into the air. Some studies indicate that this outgassing of additives may contribute to health complications, and have resulted in a call for banning the use of DEHP on shower curtains, among other uses.[31] The Japanese car companiesToyota, Nissan, and Honda have eliminated PVC in their car interiors starting in 2007.
In 2004 a joint Swedish-Danish research team found a statistical association between allergies in children and indoor air levels of DEHP and BBzP (butyl benzyl phthalate), which is used in vinyl flooring.[32] In December 2006, the European Chemicals Bureau of the European Commission released a final draft risk assessment of BBzP which found "no concern" for consumer exposure including exposure to children.[33]
EU decisions on phthalates
Risk assessments have led to the classification of low molecular weight and labeling as Category 1B Reproductive agents. Three of these phthalates, DBP, BBP and DEHP were included on annex XIV of the REACH regulation in February 2011 and will be phased out by the EU by February 2015 unless an application for authorisation is made before July 2013 and an authorisation granted. DIBP is still on the REACH Candidate List for Authorisation. Environmental Science & Technology, a peer reviewed journal published by the American Chemical Society states DEHP poses a serious risk to human health.[34]
In 2008 the European Union's Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR) reviewed the safety of DEHP in medical devices. The SCENIHR report states that certain medical procedures used in high risk patients result in a significant exposure to DEHP and concludes there is still a reason for having some concerns about the exposure of prematurely born male babies to medical devices containing DEHP.[35] The Committee said there are some alternative plasticizers available for which there is sufficient toxicological data to indicate a lower hazard compared to DEHP but added that the functionality of these plasticizers should be assessed before they can be used as an alternative for DEHP in PVC medical devices. Risk assessment results have shown positive results regarding the safe use of High Molecular Weight Phthalates. They have all been registered for REACH and do not require any classification for health and environmental effects, nor are they on the Candidate List for Authorisation. High phthalates are not CMR (carcinogenic, mutagenic or toxic for reproduction), neither are they considered endocrine disruptors.
In the EU Risk Assessment the European Commission has confirmed that Di-isononyl phthalate (DINP) and Di-isodecyl phthalate (DIDP) pose no risk to either human health or the environment from any current use. The European Commission's findings (published in the EU Official Journal on April 13, 2006)[36] confirm the outcome of a risk assessment involving more than 10 years of extensive scientific evaluation by EU regulators. Following the recent adoption of EU legislation with the regard to the marketing and use of DINP in toys and childcare articles, the risk assessment conclusions clearly state that there is no need for any further measures to regulate the use of DINP. In Europe and in some other parts of the world, the use of DINP in toys and childcare items has been restricted as a precautionary measure. In Europe, for example, DINP can no longer be used in toys and childcare items that can be put in the mouth even though the EU scientific risk assessment concluded that its use in toys does not pose a risk to human health or the environment. The rigorous EU risk assessments, which include a high degree of conservatism and built-in safety factors, have been carried out under the strict supervision of the European Commission and provide a clear scientific evaluation on which to judge whether or not a particular substance can be safely used.
The FDA Paper titled "Safety Assessment of Di(2-ethylhexyl)phthalate (DEHP)Released from PVC Medical Devices" states that [3.2.1.3] Critically ill or injured patients may be at increased risk of developing adverse health effects from DEHP, not only by virtue of increased exposure relative to the general population, but also because of the physiological and pharmacodynamic changes that occur in these patients compared to healthy individuals.[37]
Vinyl chloride monomer
Main article: vinyl chloride
In the early 1970s, the carcinogenicity of vinyl chloride (usually called vinyl chloride mononomer or VCM) was linked to cancers in workers in the polyvinyl chloride industry. Specifically workers in polymerization section of a B.F. Goodrich plant near Louisville, Kentucky (US) were diagnosed with liver angiosarcoma also known as hemangiosarcoma, a rare disease.[38] Since that time, studies of PVC workers in Australia, Italy, Germany, and the UK have all associated certain types of occupational cancers with exposure to vinyl chloride, and it has become accepted that VCM is a carcinogen.[4] Technology for removal of VCM from products have become stringent commensurate with the associated regulations.
Dioxins
Main article: Polychlorinated dibenzodioxins
PVC produces HCl upon combustion almost quantitatively related to its chlorine content. Extensive studies in Europe indicate that the chlorine found in emitted dioxins is not derived from HCl in the flue gases. Instead, most dioxins arise in the condensed solid phase by the reaction of inorganic chlorides with graphitic structures in char-containing ash particles. Copper acts as a catalyst for these reactions.[39]
Studies of household waste burning indicate consistent increases in dioxin generation with increasing PVC concentrations.[40] According to the EPA dioxin inventory, landfill fires are likely to represent an even larger source of dioxin to the environment. A survey of international studies consistently identifies high dioxin concentrations in areas affected by open waste burning and a study that looked at the homologue pattern found the sample with the highest dioxin concentration was "typical for the pyrolysis of PVC". Other EU studies indicate that PVC likely "accounts for the overwhelming majority of chlorine that is available for dioxin formation during landfill fires."[40]
The next largest sources of dioxin in the EPA inventory are medical and municipal waste incinerators.[41] Various studies have been conducted that reach contradictory results. For instance a study of commercial-scale incinerators showed no relationship between the PVC content of the waste and dioxin emissions.[42][43] Other studies have shown a clear correlation between dioxin formation and chloride content and indicate that PVC is a significant contributor to the formation of both dioxin and PCB in incinerators.[44][45][46]
In February 2007, the Technical and Scientific Advisory Committee of the US Green Building Council (USGBC) released its report on a PVC avoidance related materials credit for the LEED Green Building Rating system. The report concludes that "no single material shows up as the best across all the human health and environmental impact categories, nor as the worst" but that the "risk of dioxin emissions puts PVC consistently among the worst materials for human health impacts."[47]
In Europe the overwhelming importance of combustion conditions on dioxin formation has been established by numerous researchers. The single most important factor in forming dioxin-like compounds is the temperature of the combustion gases. Oxygen concentration also plays a major role on dioxin formation, but not the chlorine content.[48]
The design of modern incinerators minimises PCDD/F formation by optimising the stability of the thermal process. To comply with the EU emission limit of 0.1 ng I-TEQ/m3 modern incinerators operate in conditions minimising dioxin formation and are equipped with pollution control devices which catch the low amounts produced. Recent information is showing for example that dioxin levels in populations near incinerators in Lisbon and Madeira have not risen since the plants began operating in 1999 and 2002 respectively.
Several studies have also shown that removing PVC from waste would not significantly reduce the quantity of dioxins emitted. The European Union Commission published in July 2000 a Green Paper on the Environmental Issues of PVC. "[49] The Commission states (page 27) that it has been suggested that the reduction of the chlorine content in the waste can contribute to the reduction of dioxin formation, even though the actual mechanism is not fully understood. The influence on the reduction is also expected to be a second or third order relationship. It is most likely that the main incineration parameters, such as the temperature and the oxygen concentration, have a major influence on the dioxin formation". The Green Paper states further that at the current levels of chlorine in municipal waste, there does not seem to be a direct quantitative relationship between chlorine content and dioxin formation.
A study commissioned by the European Commission on "Life Cycle Assessment of PVC and of principal competing materials" states that "Recent studies show that the presence of PVC has no significant effect on the amount of dioxins released through incineration of plastic waste."[50]
End-of-life
The European waste hierarchy refers to the 5 steps included in the article 4 of the Waste Framework Directive:[51]
- Prevention - preventing and reducing waste generation.
- Reuse and preparation for reuse - giving the products a second life before they become waste.
- Recycle - any recovery operation by which waste materials are reprocessed into products, materials or substances whether for the original or other purposes. It includes composting and it does not include incineration.
- Recovery - some waste incineration based on a political non-scientific formula[citation needed]that upgrades the less inefficient incinerators.
- Disposal - processes to dispose of waste be it landfilling, incineration, pyrolysis, gasification and other finalist solutions. Landfill is restricted in some EU-countries through Landfill Directives and there is a debate about Incineration E.g. original plastic which contains a lot of energy is just recovered in energy and not recycled. According to the Waste Framework Directive the European Waste Hierarchy is legally binding except in cases that may require specific waste streams to depart from the hierarchy. This should be justified on the basis of life-cycle thinking.
The European Commission has set new rules to promote the recovery of PVC waste for use in a number of construction products. It says: "The use of recovered PVC should be encouraged in the manufacture of certain construction products because it allows the reuse of old PVC [..] This avoids PVC being discarded in landfills or incinerated causing release of carbon dioxide and cadmium in the environment".[52]
Industry initiatives
In Europe, developments in PVC waste management have been monitored by Vinyl 2010,[53]established in 2000. Vinyl 2010's objective was to recycle 200,000 tonnes of post-consumer PVC waste per year in Europe by the end of 2010, excluding waste streams already subject to other or more specific legislation (such as the European Directives on End-of-Life Vehicles, Packaging and Waste Electric and Electronic Equipment).
Since June 2011, it is followed by Vinylplus, a new set of targets for sustainable development.[54] Its main target is to recycle 800,000 tonnes/year of PVC by 2020 including 100,000 tonnes of difficult to recycle waste. One technology for collection and recycling of PVC waste is Recovinyl[55] which reported the recycled tonnage as follows: pipe 25 kT, profile 107 kT, rigid film 6 kT, flexible cables 79 kt and mixed flexible 38 kT.
One approach to address the problem of waste PVC is through the process called Vinyloop. It is a mechanical recycling process using a solvent to separate PVC from other materials. This solvent turns in a closed loop process in which the solvent is recycled. Recycled PVC is used in place of virgin PVC in various applications: coatings for swimming pools, shoe soles, hoses, diaphragms tunnel, coated fabrics, PVC sheets.[56] This recycled PVC's primary energy demand is 46 percent lower than conventional produced PVC. So the use of recycled material leads to a significant better ecological footprint. The global warming potential is 39 percent lower.[57]
Restrictions
In November, 2005 one of the largest hospital networks in the U.S., Catholic Healthcare West, signed a contract with B. Braun Melsungen for vinyl-free intravenous bags and tubing.[58]
In January, 2012 a major U.S. West Coast healthcare provider, Kaiser Permanente, announced that it will no longer buy intravenous (IV) medical equipment made with polyvinyl chloride (PVC) and DEHP (di-2-ethyl hexyl phthalate) type plasticizers.[59]







No comments:
Post a Comment